What is COVID-19?
Coronaviruses comprise a large family of viruses, some of which cause respiratory illnesses in humans, ranging from common colds to more severe conditions.1 The coronavirus involved in the outbreak that began in 2019 was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 The disease it causes was named “coronavirus disease 2019” or “COVID-19.”2
Our Commitment to Fighting COVID-19
From the beginning of the pandemic, we understood that fighting COVID-19 would require the power of science and unprecedented collaboration among scientists, companies, governments, and other stakeholders around the world.
Since 2020, we have been working on a multi-pronged approach to address COVID-19 and today we are focused on prevention through vaccination and treatment for appropriate adult patients through medication. While there have been advancements in the fight against COVID-19, there is still more work to be done.
Making the Impossible Possible
COVID-19 Vaccine: Prevention through getting eligible people vaccinated is critical in helping the fight against COVID-19. That’s why, in 2020, we set out to make the impossible possible. In collaboration with BioNTech, we delivered a breakthrough COVID-19 vaccine to the world using messenger RNA (mRNA) technology, which is a molecule that contains the instructions or recipe that directs the cells to make a protein using its natural machinery.
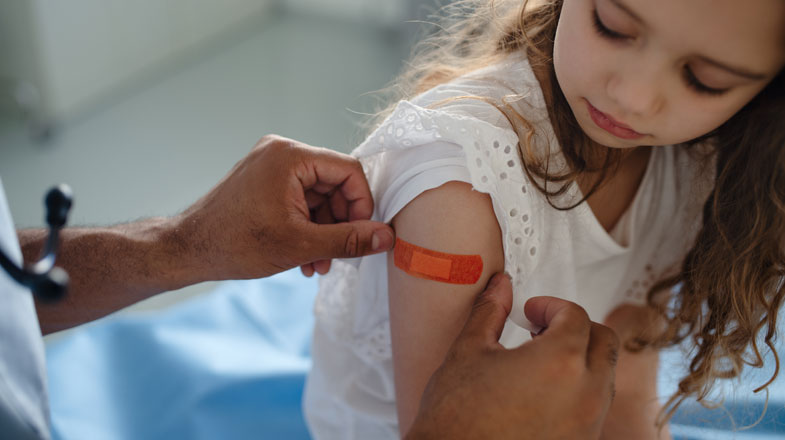
We worked with tremendous urgency, without compromising quality, coordinating closely with trial participants and investigators, regulatory bodies, other companies, and governments to bring this vaccine forward. Our success was made possible in part due to Pfizer’s longstanding history in vaccine research, development, and delivery, which dates back more than a century.
Getting Ahead of COVID-19

In May 2023, three years after COVID-19 was designated as a pandemic, the World Health Organization (WHO) and the U.S. Department of Health and Human Services (HHS) declared the end of the Public Health Emergency (PHE) for COVID-19.3,4 While clinical and real-world data still show that existing COVID-19 vaccine options can help protect against the virus, we are continuing to follow the science by exploring new vaccine approaches that may be needed as the virus evolves. We also continue to run a COVID-19 clinical drug development program, consisting of completed and ongoing clinical trials.
Our goal is to stay ahead of the virus by continuing to progress cutting-edge science that we believe may help serve patients in need.
In the meantime, we believe that one of the best safeguards against the spread of COVID-19 is getting all eligible people up to date with their vaccination schedule, and for those who do get COVID-19, educating and encouraging them to speak with their healthcare professional about available treatment options.
Frequently Asked Questions About Pfizer and COVID-19
- Why do we need both COVID-19 vaccine options and oral treatments?
While vaccines are one of the best ways individuals can help reduce the risk of infection, COVID-19 cases are still possible, and some people are at high risk for severe illness. Therefore, it is important that we have access to testing and treatment options for individuals that are diagnosed with COVID-19. This is why both vaccines and treatment options are necessary to help reduce the larger burden of COVID-19 disease in the world.
- If I’m vaccinated and have COVID-19, can I take an oral treatment?
COVID-19 oral treatments may be right for you, even if you are vaccinated, if you are at high risk of progressing to severe illness. Talk to your doctor for individual medical advice.
- Why should I get vaccinated if I am eligible to take an oral treatment option if I test positive for COVID-19?
Clinical and real-world data show that COVID-19 vaccines can help protect against COVID-19, particularly against the risk for severe disease and hospitalization. However, if you do get sick, have symptoms of COVID-19, and have a high-risk factor for progression to severe illness, you should speak with your doctor about treatment options as soon as possible.
- What are the side effects of mRNA COVID-19 vaccines?
Side effects may vary depending on the individual and which vaccine is administered. Be sure to consult a healthcare provider to make decisions that are right for your medical history.
Safety is a top concern for all of us and Pfizer takes reports of side effects that are potentially associated with our COVID-19 vaccines very seriously. While hundreds of millions of people have taken the vaccine safely, it is important to note that every medicine—and vaccine—has side effects.5 These side effects are rigorously monitored in clinical trials to ensure the benefits outweigh the risks.6
More detailed information is available on the CDC’s Possible Side Effects After Getting a COVID-19 Vaccine page. Please visit our Coronavirus Resources page for more information about Pfizer’s work on COVID-19.
Emergency Use Authorization
Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) has not been approved or licensed by FDA, but has been authorized for emergency use by FDA, under an EUA to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 6 months through 11 years of age. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b) (1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner. Please see EUA Fact Sheets at www.cvdvaccine-us.com
U.S. FDA Emergency Use Authorization Statement
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of PAXLOVID for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40 kg) with mild-to-moderate coronavirus disease 2019 (COVID-19) and who are at high risk for progression to severe COVID-19, including hospitalization or death.
PAXLOVID is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of PAXLOVID under 564(b)(1) of the Food Drug and Cosmetic Act unless the authorization is terminated or revoked sooner.
- References
- Human Coronavirus Types. Centers for Disease Control and Prevention. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/coronavirus/types.html.
- Naming the coronavirus disease (COVID-19) and the virus that causes it. WHO. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed October 25, 2021.
- World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Published May 5, 2023. Accessed May 8, 2023.
- U.S. Health and Human Services. COVID-19 Public Health Emergency (PHE). https://www.hhs.gov/coronavirus/covid-19-public-health-emergency/index.html. Updated May 5, 2023. Accessed May 8, 2023.
- Possible side effects from vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/vac-gen/side-effects.htm. Published April 2020. Accessed July 2023.
- Development & Approval Process. U.S. Food and Drug Administration. https://www.fda.gov/drugs/development-approval-process-drugs. Published August 8, 2022. Accessed July 10, 2023.















