- Science
- Clinical Trials
- Guide to Clinical Trials Your participation makes a difference
- Clinical Trials in Children Designed to improve kids' health
- Data and Results Sharing our Results
- Integrity and Transparency Building Trust
- Diversity Equity and Representation
- Plain Language Study Results Trial Result Summaries
- Expanded Access & Compassionate Use Possible Treatment Options
- Find a Trial
- Areas of Focus
- Rare Disease Smaller populations but big impact
- Internal Medicine Extending lifespans worldwide
- Inflammation & Immunology Treatment at the molecular level
- Vaccines Preventing the spread of infections
- Oncology The science of optimism
- Anti Infectives Combatting an evolving risk
- Areas of Innovation
- Gene Therapy Breakthroughs become treatments
- Medicinal Sciences The next generation of science
- Precision Medicine Developing tailored medicines
- Maternal Immunization Protecting newborns at the start
- mRNA Technology Unleashing the next wave of scientific innovations
- Diseases & Conditions
- Coronavirus Resources
- Product Pipeline
- Research Sites
- Clinical Trials
- Products
- How Drugs are Made
- Branded vs. Generic Learn the difference
- Biologics & Biosimilars Cures found in nature
- Commitment to Quality Maintaining the highest standards
- Global Supply Strategic manufacturing locations
- Manufacturing Sites Where medicine is made in the U.S.
- Medicine Safety
- Health Literacy Learning to be well
- Treatment Choices Learning about treatment decisions
- Partnering With Patients Helping others by reporting side effects
- Tips for Patients Preventing medication errors
- Reporting Adverse Events
- Counterfeiting Preventing medication errors
- Product Safety
- Product List
- Product Contacts
- PfizerPro for Professionals
- Patient Assistance Programs
- Distributors
- How Drugs are Made
- Stories
- Newsroom
- About
- People
- Executives Our senior-most leadership
- Board Members The people steering our company
- Scientists Our experts making discoveries
- Patient Stories Our patients
- Colleague Stories Our colleagues
- Responsibility
- Ethics & Compliance Each of us is responsible
- Responsible Business Breakthroughs that change patients’ lives
- Patient Advocacy & Engagement Putting Patients First
- Global Impact Meeting urgent needs worldwide
- Diversity, Equity, and Inclusion Everyone has something to offer
- Environmental Sustainability Our responsiblity to the environment
- Human Rights Furthering dignity and worth
- Health & Safety
- Intellectual Property The benefits of fair competition
- EHS Governance
- Misinformation
- Programs & Policies
- Grants Support for independent research
- Political Partnership Supporting like-minded organizations
- Working with Healthcare Professionals Collaboration to improve lives
- Prescription Value & Pricing How to lower patient costs
- Privacy Principles Commitment to personal data privacy
- Ready for Cures Improving Access to Medicines
- Transparency in Grants Committed to Disclosure
- Policy Positions
- Investors
- Investors Overview Information for stockholders
- Why Invest Why to join us in our mission
- Events & Presentations Calendar of upcoming events
- Financial Reports Quarterly reports and more
- Investor News Announcements about our performance
- Stock Information Charts and data
- Shareholder Services Information on stock transactions
- Corporate Governance
- Corporate Governance Overview Gaining insight into our performance
- Board Committees & Charters Defining the corporate structure
- The Pfizer Board Policies Ensuring ethical leadership
- Corporate Governance FAQs Learn more about our approach
- Contact Our Directors Email any of our Directors
- Purpose
- History
- Careers
- Partners
- People
Let’s Protect Patients From the Pill Penalty
Congress passed the Inflation Reduction Act (IRA) with the intent of helping Americans facing the highest inflation rates in decades, but the law’s government price-setting provision for medicines covered by Medicare could have negative consequences for patients.
Specifically, the IRA created a “pill penalty” that could discourage the development of medicines that typically come in pill or capsule form. It allows the government to start the price-setting process for pills and capsules seven years after small molecule medicines (e.g., tablets, capsules, and pills) are initially approved by the Food and Drug Administration (FDA). This time frame is roughly half of the current average time frame of 13–14 years before patents and exclusivities expire on medicines and generic competitors emerge. The significantly shorter time frame discourages innovation and risks leaving patients without the treatment options they desperately need.
Consequences of this pill penalty include:
Less access to the benefits of small molecule medicines. They’re small, but mighty: Small molecule medicines have significant therapeutic benefits for hard-to-treat diseases like cancer. They also have a unique ability to reach therapeutic targets inside the brain, which means they serve a critical role in the treatment of health conditions affecting the central nervous system, such as mental illness, stroke, epilepsy, and various neurodegenerative diseases.
In addition, because small molecule medicines come in a variety of pill forms, such as tablets or capsules, they are easier to take and patients often prefer them. Having the option of a pill form may improve adherence, potentially keeping patients healthier.
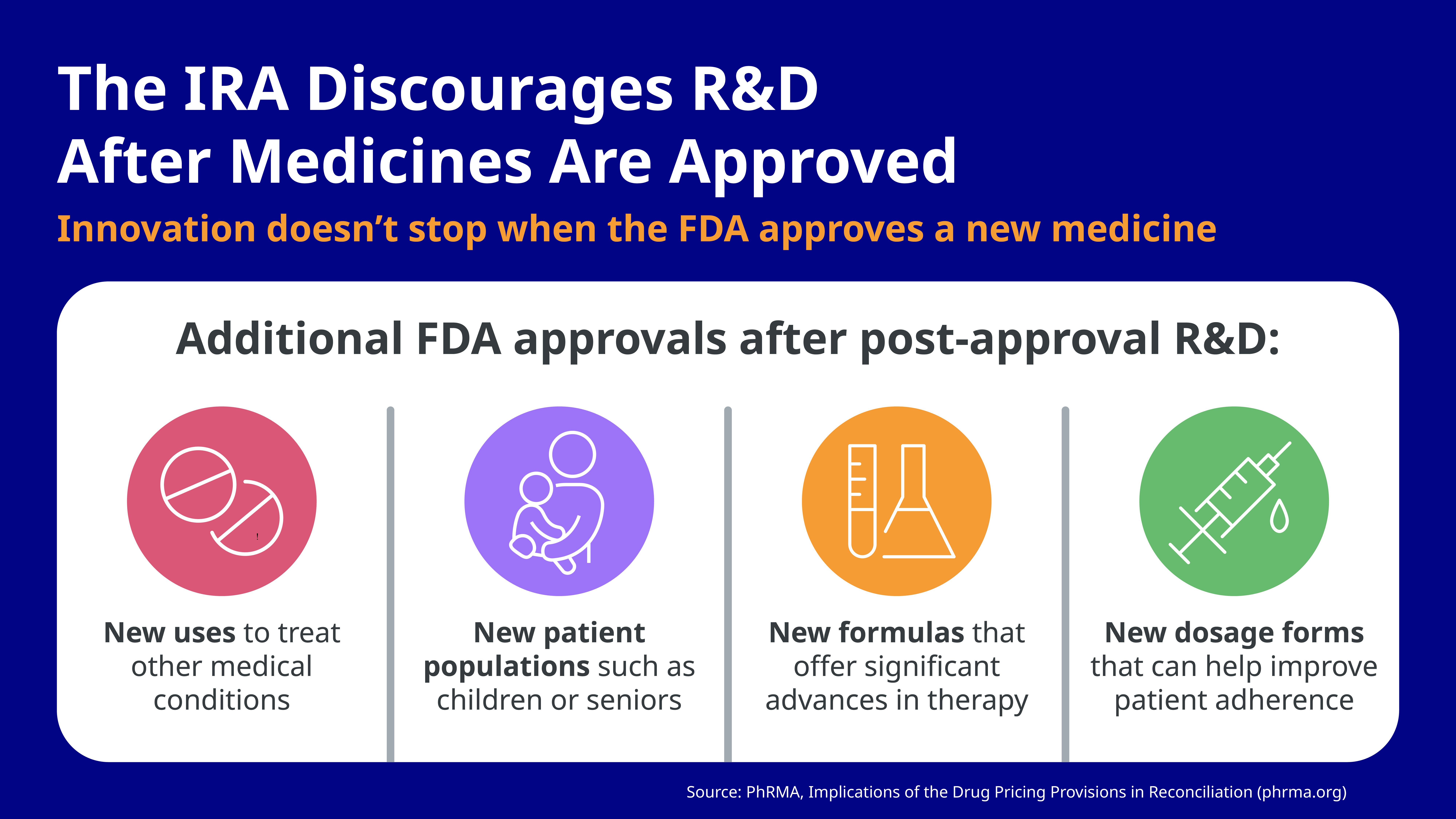
Fewer new medicines for patients who need them. Innovation doesn’t stop when the FDA approves a new prescription medicine. Biopharmaceutical companies continue to research treatments after FDA approval, as researchers learn more and follow scientific leads after initial approval to see if these treatments can be used in new ways to improve patient care. The investments in research and development (R&D) after medicines are approved represent important advances for patients, including the development of new uses to treat a different medical condition, new patient populations such as children or seniors, new formulas, and new dosage forms.
Recent research shows how the pill penalty can discourage critical post-approval R&D — hindering innovation to fight cancer and rare diseases and blocking other treatment advances. For example:
- One study of small molecule medicines approved from 2006 through 2012 found that more than half received at least one additional indication after the initial FDA approval. Of these post-approval indications, 45% were approved seven or more years after initial approval and 63% were approved five or more years after approval.1
- Another study estimates that as many as 139 drugs over the next 10 years are at risk of not being developed because of the dangerous price-setting policies in the IRA.2
- In addition, there will be an estimated 40% reduction in new medicines being brought to market in the next 10 years.2
Less research and innovation to help cure patients with cancer. Much of the progress made in the fight against cancer is the result of research conducted on cancer medicines after the FDA first approves them. In fact, more than 60% of oncology medicines approved a decade ago received approvals for additional indications in later years, and most of those were seven or more years after initial FDA approval.3 The price-setting provisions in the IRA put this type of research on new uses for existing cancer medicines at risk.
In addition, small molecule medicines are an important part of the treatment arsenal for cancer because they can be administered orally and can enter cells to reach cancer targets. Small molecule oral-targeted therapies work by interfering with the processes that enable tumors to grow and spread throughout the body. These therapies make up the majority of cancer medicines approved. Having a broad range of both small molecule medicines and biologics is an important part of the effort to drive down cancer mortality in the years ahead.
Less research and innovation to address America’s mental health crisis. America is facing a mental health crisis, and the IRA will likely hinder the nation’s ability to overcome it. Development of new medicines to treat mental illnesses has significantly lagged behind advancements made in other areas over the past 30 years. Treatments for mental illnesses are some of the most difficult to develop — with long clinical development timelines and low success rates.
Small molecule medicines are the primary medicines used to treat patients with mental illness because they have the unique ability to reach therapeutic targets inside the brain. Despite the critical importance of these medicines to patients with mental illness, the IRA will discourage the R&D needed to bring forward new and improved treatments. For companies with R&D projects in areas such as mental health, 82% expect the IRA to have substantial impacts on their pipeline.4
- Job loss. Research estimates that the IRA’s pill penalty could result in a loss of 342,000–676,000 indirect jobs in the U.S. biopharma ecosystem.2
Post-Approval Advances Should Be Encouraged, Not Discouraged
Congress should prioritize preserving innovation in the development of life-saving treatments, and that starts with fixing the pill penalty so that small molecule medicines are not eligible for price-setting before they have been on the market for at least 11 years, with the price taking effect two years later.
Join Pfizer’s Ready for Cures
Stay up to date: Sign up below to join Pfizer’s Ready for Cures community to stay up to date on the IRA and learn about smart policies that can help support medical innovation and increase patients’ access to the medicines they need.
- Partnership for Health Analytic Research. “Implications of the Inflation Reduction Act price setting provisions on post-approval indications for small molecule medicines,” June 2023. https://www.pharllc.com/wp-content/uploads/2023/05/Implications-of-the-IRA-on-Post-Approval-Small-Molecules-2006-2012_Final.pdf
- Vital Transformation. “IRA’s Impact on the US biopharma ecosystem,” 2023. https://vitaltransformation.com/2023/05/iras-impact-on-the-us-biopharma-ecosystem
- Partnership for Health Analytic Research. “Implications of the Inflation Reduction Act on Post-Approval Research & Development of Biopharmaceutical Medicines,” November 9, 2022. https://www.pharllc.com/wp-content/uploads/2022/11/Clinical-Benefits-of-Post-Authorization-Research-Brief.pdf
- PhRMA. “WTAS: Inflation Reduction Act already impacting R&D decisions,” January 17, 2023.
