Uncovering New Biomarkers to Better Understand Friedreich's Ataxia
Friedreich's Ataxia (FA) is a debilitating rare genetic disorder that causes progressive nervous system damage and movement problems. Often starting in childhood, the early symptoms include difficulty walking, poor balance, and slurred speech. Over time, people with the condition can lose sensation in their arms and legs, have problems writing and picking up objects, and experience vision and hearing loss. Many people with FA eventually need to use a wheelchair and develop life-threatening heart problems. It’s the most common form of inherited ataxia — loss of muscle control — in the U.S., affecting about one in 50,000 people.
With no current treatment options for the condition, the Friedreich's Ataxia Research Alliance (FARA), other patient advocacy groups, and the FA patient community are working closely with scientists to help better understand the disease and to hasten development of potential therapies. Pfizer scientists in collaboration with researchers in the UK have made recent advances in developing a new biomarker to help monitor the disease’s progression. For scientists developing drugs, a biomarker is a clue, or piece of evidence (such as enzyme levels or heart rate), that can help measure how a patient responds to a medicine.
As Pfizer is currently in the pre-clinical stages of developing a potential gene therapy to treat FA, a better biomarker may help scientists run studies faster and more efficiently. “The key issue with gene therapy is that because it's a one-time treatment, you only get one chance to get the dose right,” says Lawrence Charnas, MD, FA Clinical Lead at the Rare Disease Research Unit at Pfizer. “Testing in the least number of patients, you want to make a decision whether you need to increase the dose. This biomarker we’re looking at is intended to help us make that decision as quickly as possible,” he adds.
Frataxin and iron metabolism
FA is caused by a mutation in the frataxin gene that codes for a protein (by the same name), which is important for the functioning of mitochondria, the cell’s energy powerhouse. To develop the condition, most people must inherit a copy of the mutated gene from both parents.
The mutation disrupts the normal production of the frataxin protein in cells. While the exact function of frataxin is not fully understood, researchers know that it plays an important role in helping the mitochondria use iron as a part of the cell’s metabolic processes. Normally, when iron enters the mitochondria, frataxin helps form metabolites called iron-sulfur clusters, which are necessary for energy production. But without sufficient levels of frataxin, the mitochondria are not able to provide sufficient amounts of the iron-sulfur clusters, hence affecting the cell energy powerhouse.
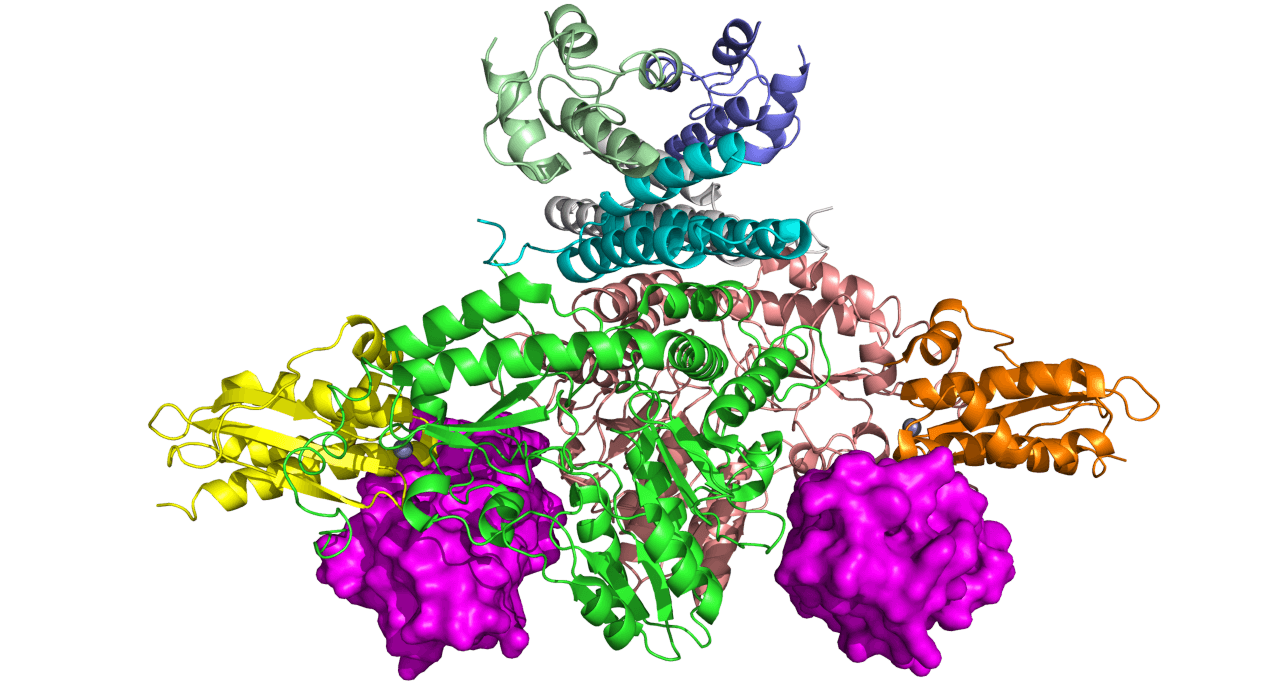
As a part of the efforts to understand the function of frataxin, scientists have recently produced a cryogenic electron microscopy (cryo-EM) image of the frataxin protein while performing its function. “By understanding this structure, scientists can have a better understanding of how frataxin is involved in producing iron-sulfur clusters, which are important for the mitochondria for energy production and metabolism,” says Alain Martelli, Senior Principal Scientist in the Rare Disease Research Unit at Pfizer’s Kendall Square, Cambridge, Mass. research site. “This emphasizes our commitment to not only understand the disease, but the biology behind it,” he adds.
Advances in biomarkers
Historically, it’s been difficult to develop biomarkers for FA because the frataxin protein stays inside cells and is not excreted. While the disease can be diagnosed by testing for genetic abnormalities circulating in the blood, to understand the extent of damage at the organ level, scientists need another measure. “Since frataxin is only in the cell, it's really hard to understand how having depleted levels impacts the tissues and ultimately causes people’s symptoms,” says Charnas.
For the new biomarker, scientists are focusing on mitochondrial complex I (MC1), a complex of the mitochondria that requires iron-sulfur clusters and is involved in the production of energy for the cell. Scientists believe that measuring MC1 levels can help measure early signs of frataxin deficiency.
In addition, Pfizer scientists are working to develop digital wearables, which can be used to help study FA patients at home during clinical trials. These digital tools can be used to track patients’ movement, which is a biomarker to evaluate the progression and severity of their condition. The FA patient and advocacy community have been valuable collaborators in helping us better understand and advance these tools. “It helps to make participation in clinical trials easier and to have more robust measures of function that would potentially enable faster and smaller trials,” says Charnas.





