Patients as Partners in Developing New Medicines

With a growing emphasis on patient-reported outcomes, study volunteers are becoming active participants in evaluating a treatment’s efficacy.
Since the first reported controlled clinical trial in 1747, when Scottish surgeon James Lind studied how eating oranges and lemons can cure scurvy among sailors at sea, patients have always been essential to expanding medical knowledge and developing new therapies. After all, no one knows your body like you do.
But for the most part, patients have been silent partners in the study process. Clinicians gather patient data through blood tests and other diagnostic tools and record their reports of symptoms.
But in recent years, there has been a greater push to include patient’s feedback in clinical research. These volunteers are often very willing to report their symptoms and physical and emotional states and welcome the opportunity be more actively involved in the pursuit of new treatments.
It’s by no means meant to supplant traditional, objective data gathering, but rather to complement this information.
As part of the wider push across health care for more patient-centered care, patient-reported outcomes (PROs) is an umbrella term used to describe any data reported directly by the patient, ranging from side effects (such as pain and nausea), to daily functioning, to quality of life.
Technological advances are fueling this shift. From cloud computing software that incorporates patients’ electronic-reported outcomes to the use of mobile apps and tablets, patients can become more active participants in the study process even when they’re outside of the clinic. In one recent cancer study, patients had the ease of reporting their symptoms via emojis on smartwatches, replacing traditional paper questionnaires.
Voices of Patients With Chronic Disease
PROs have an established track record in the study of chronic diseases such as lupus and rheumatoid arthritis, where the impact of treatment may not result in a durable cure, but improves functioning and quality of life.
In one recent partnership, Pfizer joined forces with the Lupus Research Alliance to create a mobile app that might eventually allow patients participating in clinical trials to record their symptoms in real time. Traditionally, patients reported symptoms during doctor’s visit and relied on memory. But for inflammatory conditions like lupus, pain and fatigue episodes known as “flares” strike unpredictably and often far before the doctor visit. Enabling patients to report symptoms “in the moment” provides the potential for more robust, accurate, and comprehensive data.
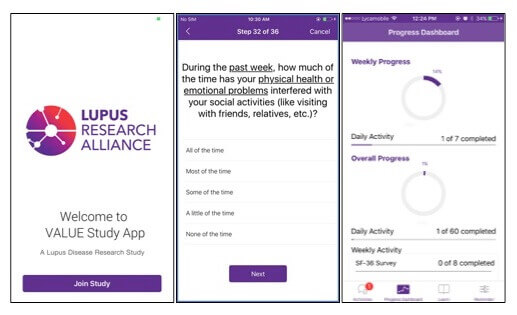
Pfizer recently joined forces with the Lupus Research Alliance to create a mobile app that lets patients record their symptoms in real time.
In another example, PRO data from a Pfizer RA treatment was eventually used on the approved treatment’s label. For patients taking a medication, this adds more information about potential side effects and what to expect during treatment.
Tech and PRO, For Better Cancer Care
Similarly, for cancer patients who are weighing a variety of complex treatment options (such as surgery, chemotherapy, radiation, hormonal therapy and immunotherapy) that come with risks and side effects, PRO data from their “peers” can help them make better-informed decisions about what to expect.
Regular symptom monitoring during cancer care can also help improve patient’s outcomes.
One recent study published in the Journal of the American Medical Association, found that patients who reported their symptoms in “real-time,” while receiving chemotherapy reported higher quality-of-life outcomes and survival rates.
And cancer patients, despite experiencing a variety of debilitating symptoms, are willing and eager to report PROs, according to a June 2017 study in the International Journal of Radiation Biology, Oncology, Physics. "Patients who are participating in clinical trials are enthusiastic about providing information about how they're feeling, and they value the opportunity to have that information become part of the study," said the lead investigator, Ethan Basch, M.D., of the Lineberger Comprehensive Cancer Center at the University of North Carolina, in a recent National Cancer Institute article.
For patients across all conditions, including their feedback in clinical trials welcomes a new era with richer data and more personalized therapies.