5 Things Worth Knowing About Biosimilars and Interchangeability
Biologic drugs have revolutionized disease management for many serious and chronic conditions including cancer, rheumatoid arthritis, Crohn’s disease, and psoriasis.1 Versions of biologic drugs, known as biosimilars, have helped improve access to these critical medicines for a wider patient population and lowered healthcare costs.2
As biosimilars continue to hit the market in greater numbers, you’ll be hearing more about this class of drugs, as well as the “interchangeability” designation that some biosimilars receive.
Here are five things you need to know about interchangeability, as well as short videos and an interactive map to help you explore this topic.
1. Some biosimilars have an additional regulatory designation called interchangeability
In the U.S., a biosimilar with an “interchangeable” designation can be substituted for its reference medicine at the pharmacy, without additional approvals from the prescribing physician, state law permitting. Sound familiar? This is similar to the way that a pharmacist can substitute a generic medicine for a branded drug when filling a prescription.
To achieve an interchangeable designation, clinical studies are conducted to demonstrate that there is no additional risk or reduced drug effectiveness if a patient switches back and forth between an interchangeable biosimilar and a reference medicine, as compared to receiving treatment with just the reference medicine.3
These switching studies are conducted in addition to the rigorous approval process that all biosimilars undergo to meet the exacting FDA standards for biosimilar safety and efficacy.4
To learn more about interchangeability and the evidence supporting this designation, check out the videos below.
So, what might interchangeability mean for you, whether you’re a patient, physician, nurse, or pharmacist in the U.S.?
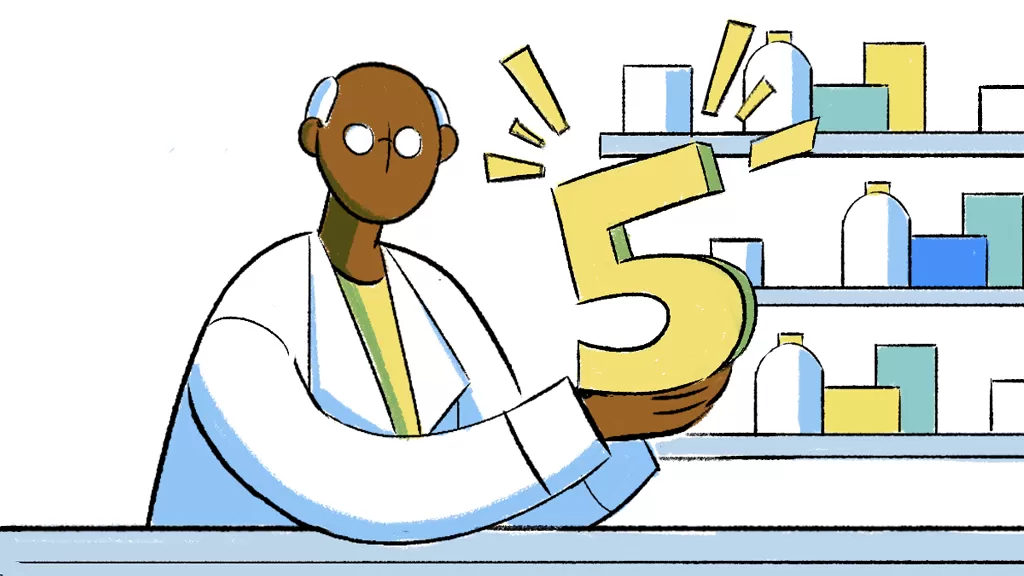
2. Interchangeability remains the exception, not the rule, when it comes to biosimilars.
The Biologics Price Competition and Innovation Act (BPCIA), which was enacted in 2010, allowed an interchangeability designation for FDA-approved biosimilars that met additional requirements.5 As of November 2023, seven biosimilars had received interchangeability designations: two insulin products6,7, three medicines used to treat certain inflammatory diseases,8,9,10 and two treatments for retinal patients.11,12 Eventually, there may be more. Other pharmaceutical companies have submitted applications seeking interchangeability designations.
3. An interchangeability designation is unique to biosimilars in the U.S.
Although biosimilars are among the fastest-growing products in the prescription drug industry, real-world experience with interchangeability is in its infancy, and therefore different countries have different regulatory approaches. For example, a separate regulatory designation for interchangeability has been instituted in the U.S.13,14,15
In the European Union, which introduced and adopted biosimilars before the U.S. did, decisions regarding pharmacy level substitution lie with each member state.16 And in Japan and Iran, a pharmacist is automatically allowed to perform a substitution when the biosimilar is approved.17 Similarly, the standards for regulatory review of biosimilars may also vary.
4. Interchangeability is granted at the federal level but governed by state laws
Biosimilars receive an interchangeability designation on a federal level from the FDA, but the local dispensing and substitution of drugs at the pharmacy level is governed by state laws. The majority of states allow reference medicines to be substituted by interchangeable biosimilars without seeking the prescribing physician’s permission, with varying degrees of notification to the prescriber and the patient. As of August 2023, 47 states and Puerto Rico allow substitution of an interchangeable biosimilar for the prescribed reference medicine without requiring prescriber permission.18,19
5. Interchangeability is significant for medicines dispensed at the pharmacy
Biologics (and biosimilars) are typically administered either by injection or intravenously. Generally, those administered by an injection are dispensed from a pharmacy and may be self-administered, while biologic medicines that are administered intravenously are often given by a healthcare professional.18,20
So, for patients who receive their biologic from a pharmacy, a reference medicine may be substituted with an interchangeable biosimilar, which may provide cost savings.6
This article is for informational purposes only and is not intended to provide legal advice.