The Future of IBD Care: Pfizer Leads a New Era of Noninvasive Monitoring
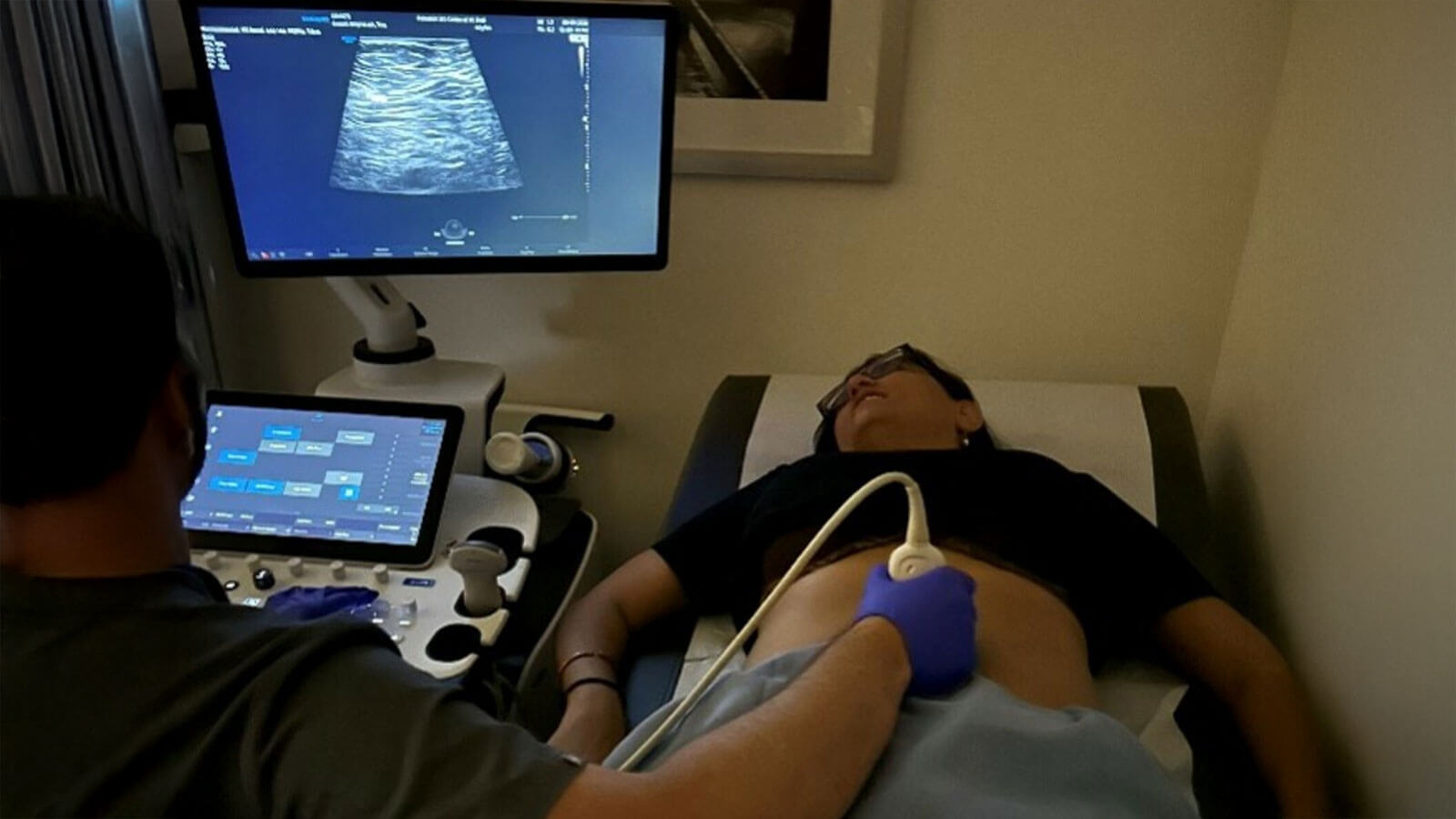
At Pfizer, we are committed to improving care and addressing inequities for patients living with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease. And, for people living with IBD, regular monitoring of the bowel is important for tracking the disease activity and severity and adjusting treatments and care as needed.1 A colonoscopy—a procedure where doctors use a flexible camera to look inside a patient’s colon—has long been the most common method of monitoring.2
However, colonoscopies can be unpleasant for patients, many of whom find them to be invasive, expensive, and time-consuming.3 For two to three days before the procedure, patients are required to restrict their diet, skip meals, drink lots of clear liquids, and cleanse their colon through medication.4 On the day of the procedure, they may go under sedation or anesthesia and have a subsequent recovery period, potentially causing them to miss work and other activities.2
While a colonoscopy is essential for diagnosis and effective for determining disease progression, many people delay or avoid the procedure altogether due to the burden of preparation, discomfort with the invasive nature of the process, fear of going under sedation or anesthesia, or anxiety over potential results.3 Avoiding colonoscopies can cause problems for the patient by delaying a diagnosis or necessary changes to their current treatment.
Another Option
Intestinal ultrasound (IUS) offers doctors a noninvasive, real-time method for examining IBD patients. IUS creates accurate images of a person’s bowel through an external device, similar to an ultrasound during pregnancy.5 Unlike colonoscopies, IUS requires no fasting, sedation/anesthesia, or insertion of a camera, making it a more convenient and accessible option for IBD monitoring (a colonoscopy is still required to diagnose IBD).5 The procedure also allows patients to discuss the results with their doctor in real time, potentially leading to quicker active disease identification and care plan implementation.5
This option, which is not as difficult as a colonoscopy in terms of preparation and cost, allows for more frequent monitoring, earlier changes to treatment, and greater patient involvement in care decisions—helping foster stronger doctor-patient relationships.
A Leading Role for Pfizer
Pfizer is committed to championing widespread adoption and use of IUS. While IUS is already a routine practice in some areas of the world—and endorsed by the European Crohn’s and Colitis Organization (ECCO)—its broader global uptake remains a key priority.
To accelerate this shift, Pfizer has helped launch and is a founding sponsor of PRISM, a groundbreaking group of healthcare providers, patients, professional societies, and industry leaders to make IUS more widely accessible worldwide. Through collaboration and innovation, we hope to pave the way for a future where IUS becomes an essential tool in the management of IBD, helping to raise the standard of care and the standard of caring for patients.
PRISM launched at this year’s 20th annual ECCO meeting in Berlin, where the first team gathering took place between leading organizations, including the International Bowel Ultrasound Group (IBUS), Intestinal Ultrasound Group of the United States and Canada (iUSCAN), and other key stakeholders.
The meeting served as a dynamic forum for exchanging innovative ideas and strategies, with discussions focused on overcoming both logistical and clinical challenges and on the formation of pilot centers for IUS implementation in the US, UK, and Canada.
“I’ve seen first-hand the impact of offering patients this non-invasive method of disease monitoring in terms of patient comfort. However, widespread adoption remains slow in part due to lack of awareness of an alternative to colonoscopies for monitoring. My hope is that the PRISM consortium will help raise awareness and encourage global use of IUS,” said Kerri Novak, Associate Professor of Medicine at the University of Calgary and President-Elect of the Intestinal Bowel Ultrasound Group.
A growing number of global healthcare leaders are investing significant time and research into IUS, and it is widely regarded as the future of IBD monitoring. Pfizer’s steadfast commitment to this innovative field is forging new partnerships with international organizations and HCPs, all with the shared goal of elevating the standard of care for people living with IBD.
“Intestinal ultrasound has immense potential to transform patient care in IBD by providing a differentiated approach to routine bowel monitoring,” said Maria Rigoroso-Brandt, Global and US Patient Engagement Lead for Inflammation and Immunology at Pfizer. “We are proud to be at the forefront of driving this innovation through our support of the PRISM initiative and are looking forward to partnering closely with global experts and patient advocates on this journey.”
“Having undergone intestinal ultrasound to monitor my Crohn’s disease, I am very encouraged that the future of IBD care is not only bright, but that it is patient-centric,” said Tina Aswani-Omprakash, CEO and co-founder, South Asian IBD Alliance. “IUS is a great tool for intermittent monitoring of disease and allows us patients and our loved ones to visualize inflammation and IBD-related complications in real time, so that we can make expedient shared decisions about our treatment modalities with our healthcare providers.”
As these partnerships evolve, the momentum behind IUS is set to redefine IBD monitoring, paving the way for more effective patient-centered care worldwide.
References
1. Vitello, A., Maida, M., Shahini, E., Macaluso, F. S., Orlando, A., Grova, M., Ramai, D., Serviddio, G., & Facciorusso, A. (2024). Current Approaches for Monitoring of Patients with Inflammatory Bowel Diseases: A Narrative Review. Journal of clinical medicine, 13(4), 1008. https://doi.org/10.3390/jcm13041008
2. Crohn’s and Colitis Foundation. How is IBD Diagnosed? https://www.crohnscolitisfoundation.org/patientsandcaregivers/what-is-ibd/diagnosing-ibd. Accessed on March 5, 2025.
3. Trevisani, L., Zelante, A., & Sartori, S. (2014). Colonoscopy, pain and fears: Is it an indissoluble trinomial? World journal of gastrointestinal endoscopy, 6(6), 227–233. https://doi.org/10.4253/wjge.v6.i6.227
4. Crohn’s and Colitis Foundation. Preparing for your Colonoscopy. https://www.crohnscolitisfoundation.org/preparing-your-colonoscopy. Accessed on March 5, 2025.
5. University of Chicago Medicine. Intestinal Ultrasound for Inflammatory Bowel Disease (IBD). https://www.uchicagomedicine.org/conditions-services/inflammatory-bowel-disease/treatment/intestinal-ultrasound-for-inflammatory-bowel-disease-ibd. Accessed on March 5, 2025
04.29.2025
04.28.2025
04.24.2025
04.17.2025
