EHS Management Systems
Overview
The foundation of Pfizer’s Environment, Health & Safety (EHS) program is robust EHS management systems. Pfizer EHS programs, applicable to all operations globally, place an emphasis on identifying and managing EHS risk. The programs are described within Global EHS Standards structured very similarly to the ISO 14001 framework with implementation at our sites verified through the Pfizer internal EHS audit program.
Pfizer’s EHS Management Systems (EHSMS) framework is risk-based and designed to meet Pfizer’s continually evolving and dynamic business and operating model. The risk-based approach offers flexibility, within defined boundaries, to apply alternative solutions that deliver equivalent levels of protection. The framework describes mandatory controls where there is potential for high consequence events and also presents risk control measures aligned with contemporary industry practices.
Pfizer's Global EHS team are responsible for developing the standards and supporting implementation documents that are the key components of Pfizer’s EHSMS. These documents are designed to protect the environment and the health and safety of our colleagues and the communities in which we operate by establishing consistent risk thresholds while allowing our operations flexibility to make decisions to manage risk most effectively. The following diagram illustrates the EHSMS documentation structure:
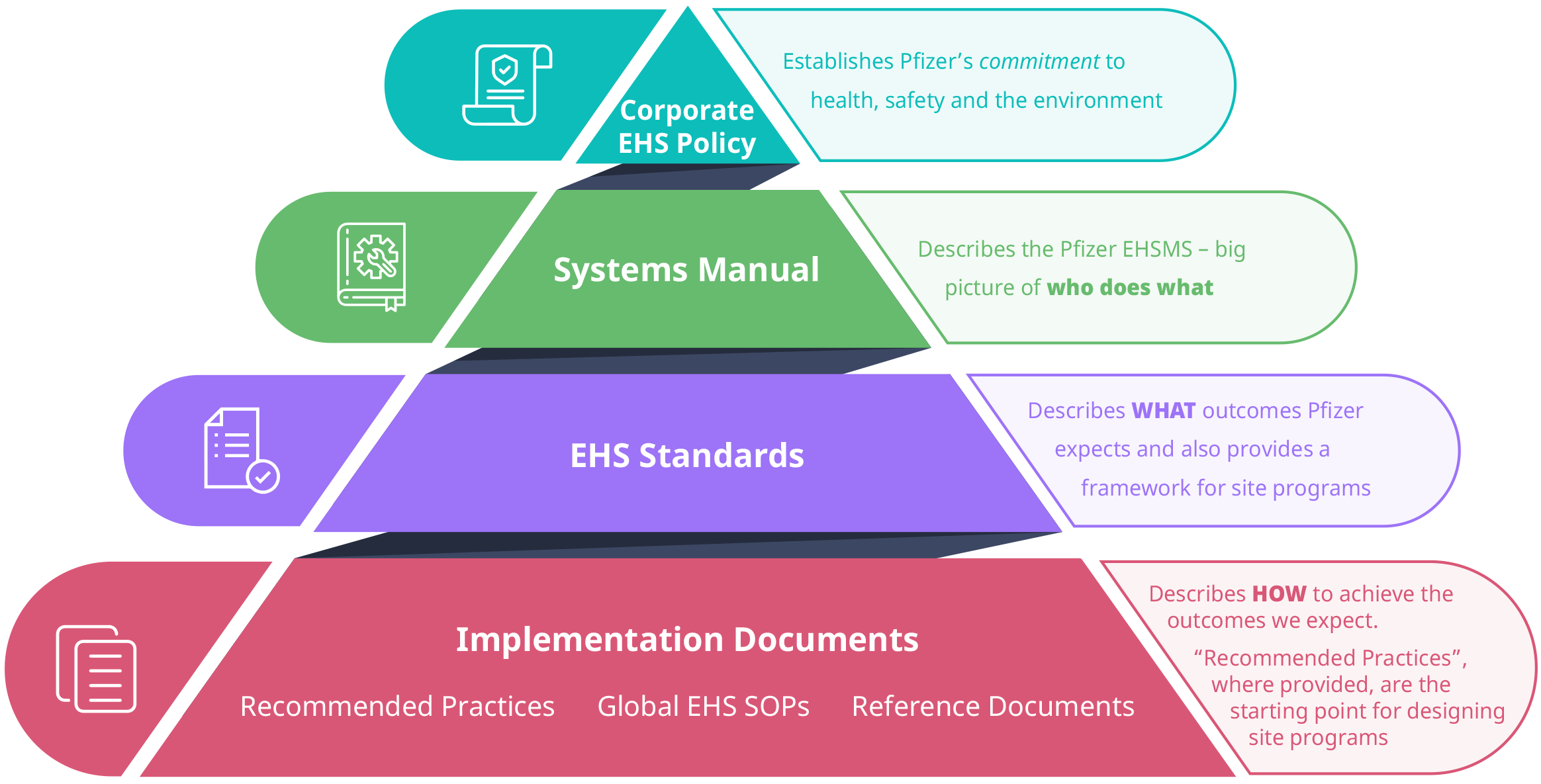
Corporate EHS Policy
Pfizer’s Corporate EHS Policy is positioned at the top of the structure. The policy establishes the company’s overall commitment to protect the health and safety of colleagues and protect the environment, while achieving high standards of Environmental, Health and Safety performance.
Systems Manual
At the next level, the Systems Manual presents a high level overview of the EHSMS.
EHS Standards
Pfizer’s EHS Standards make up the third tier of the documentation structure. A list of list of Pfizer’s Environment, Health, and Safety (EHS) Standards can be found on page 5 of this document. The Standards are arranged into four categories:
These Standards provide the framework for EHS risk management, incorporating many key elements to successful EHS management such as regulatory compliance, risk assessment, communication, self audit, and senior leadership engagement.
This group of documents sets expectations for management and control of many important EHS programs including workplace safety, occupational hygiene, fire and life safety, and environmental impact reduction. These Standards describe Pfizer’s performance expectations for these programs and include mandatory requirements where appropriate.
These Standards address key program areas across Pfizer businesses such as office safety, fleet operations, contractor safety and EHS risk from suppliers of materials and services.
These Standards ensure our operations maintain appropriate programs and processes to protect the continuity of critical activities necessary to deliver safe and effective products to patients.
Pfizer’s Management Systems Standards (Series 100) are based on and aligned with the Plan-Do-Check-Act model. These Standards require facilities to:
- Assess and prioritize risks
- Establish goals to address highest priority risks and opportunities
- Document the processes used to accomplish those goals
- Evaluate progress and adjust processes as needed to address issues and ensure continual improvement
The Plan-Do-Check-Act model of Pfizer‘s EHSMS conforms to external management system recognition standards such as ISO 14001, ISO 45001, and OHSA VPP and is considered at least equivalent to these standards.
Pfizer recognizes that external certifications (for example ISO 14001, ISO 45001, OSHA VPP) play a role in assuring performance, but certification does not benefit all sites equally. We evaluate on a case by case basis to determine whether certification would bring benefit to a site. Our internal EHS audit program is used to inform these decisions, and provide assurance of robust EHSMS at all sites.
Pfizer leadership is accountable for ensuring compliance with EHS Standards. Leadership teams are responsible for assigning responsibilities and providing resources to ensure compliance with performance expectations.
Business units, divisions and facilities are responsible for implementing EHS Standards applicable to their operations (by referring to the Scope and Applicability sections of the Standard).
Implementation Documents
Implementation Documents make up the fourth and final tier of the structure. These documents, known as Recommended Practices, Reference Documents and Global Standard Operating Procedures (SOPs), describe practical and recommended methods for conforming to EHS Standards.
Recommended Practices (RPs) are:
- Developed for situations that present significant risk to Pfizer, where, if not well-managed, the consequences of an incident would be severe (e.g., fatality, significant health impact, release, fire/ explosion leading to significant liability, harm to reputation, or business interruption) and where approaches/techniques are established and proven to be effective
- Created by technical experts and subjected to a formal review and approval process
- Mandatory (subject to the exception criteria below) because they are the accepted method for managing
key risk areas
Exception: Operations wishing to adopt an alternative to a Recommended Practice must employ a risk based decision making process to verify that the alternative achieves comparable control of risk.
Reference Documents are:
- Provided by the relevant Communities/Networks of Practice as aids to program implementation for particular EHS Standards
- Good practices; operations are not required to implement program elements and/or performance requirements included in Reference Documents
Global EHS SOPs are:
- Detailed specifications that support Global EHS/Risk Management programs
Each component of the system is designed to work interdependently in an integrated manner that continually reinforces the common objective of improving EHS performance. To ensure sustainability of the EHSMS, Pfizer facilities are required to establish formal processes for core system components and to evaluate their effectiveness regularly.
Note: The following table is a list of Pfizer’s Environment, Health, and Safety (EHS) Standards as of August 2021.
Pfizer EHS Standards support the overarching EHS Management System of governance and are arranged in four categories:
- 101 Compliance
- 102 Risk Assessment
- 103 Objective and Target Setting
- 104 Competency and Training
- 105 Communications and Consultation
- 106 Management of Change
- 107 Operational Control
- 108 Monitoring and Measurement
- 109 Self-Audit
- 110 EHS Program Review
- 111 Emergency Preparedness
- 201 Ergonomics
- 202 Safe Storage of Materials and Substances
- 203 High Hazard Work Activities
- 204 EHS Risk Management in Providing and Using Work Equipment
- 205 Safe Workplace
- 206 Fire and Life Safety
- 207 Process Safety
- 208 Occupational Hygiene
- 209 Occupational Medical Support
- 210 Environmental Impact Reduction
- 211 Ozone Depleting Compound and Refrigerant Management
- 212 Waste and Surplus Material Management
- 213 Ground and Surface Water Protection
- 214 Biological Safety
- 301 Management of EHS Risk from Suppliers of Materials and Services
- 302 Office EHS
- 303 Fleet Safety
- 304 Laboratory EHS
- 305 Acquisition and Divestiture of Real Property and Businesses
- 306 Contractor Safety
- 307 Transportation of Dangerous Goods
- 308 Controlled Substances
- 309 Labor and Ethics - Suppliers
- 401 Business Continuity Management Programs & Plans
- 402 Loss Prevention
