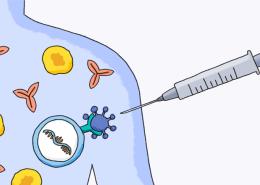
What Does mRNA Mean for the Flu Vaccine?
Throughout the COVID-19 pandemic, messenger RNA, or mRNA, has been in the spotlight for the critical role it’s playing in the first-ever approved mRNA vaccine.1 But in fact, Pfizer and BioNTech had entered into a worldwide collaboration agreement in 2018 to work on an mRNA vaccine for a different virus.
“Pfizer's first partnership with BioNTech was to look at ways to develop a more effective flu vaccine,” says John McLaughlin, who is Vice President, COVID-19/Flu Vaccines & Antivirals Lead with Pfizer.
This work has continued, and in September 2022, Pfizer began recruiting volunteers to participate in its Phase 3 clinical trial for that mRNA flu vaccine candidate. The hope, says McLaughlin, is that scientists can develop a flu vaccine faster, and with more accurate strain matching with in-season circulating strains than those currently available. One that may also spark a more robust immune response.
“As these viruses continue to adapt, what really matters is how well your vaccine matches what strains are currently circulating,” says McLaughlin. “And the speed with which you can keep up with that determines the success of a vaccination program.”
How current flu vaccines work
Today, the flu vaccine development process can be lengthy. Throughout the year, 144 national influenza centers around the world, which make up the WHO Global Influenza Surveillance and Response System (GISRS), monitor the flu viruses that are circulating before flu season begins.2 In February, the WHO makes a recommendation on the composition of flu vaccines for the upcoming fall/winter flu season in the Northern Hemisphere. That recommendation is typically based on how sick flu viruses are making people and how quickly the flu is spreading, among other factors.2 In the United States, for example, the Food and Drug Administration (FDA)’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) typically makes that decision in February or March.2
From there, it generally takes at least six months to develop and manufacture the large quantities of flu vaccines needed.2 That’s because the most common flu vaccines depend on cell growth, with the candidate vaccine virus being injected into eggs, where it replicates.3 Two other types of flu vaccines are also available. Cell-based vaccines use cultured mammalian cells instead of eggs.3 And recombinant flu vaccines use synthetic versions of the virus and are produced in insect cells.4
Unlocking the potential of an mRNA flu vaccine
mRNA vaccines, which have been in research and development for decades,5 can be made quickly and in large batches in a laboratory.5 Rather than using the virus itself, mRNA vaccines use the genetic code of a virus. That genetic code instructs the body to make proteins (called antibodies), which can help protect people from getting sick or experiencing serious illness.5
This could be a game-changer for a flu vaccine, says Verna Welch, Senior Director, mRNA Pipeline (Non-COVID) Medical and Scientific Affairs Lead. By using mRNA technology, scientists may be able to develop and manufacture the flu vaccine faster. This is, in part, because they wouldn’t have to grow the cells to make the vaccines. “If we use mRNA technology, then we may be able to develop the vaccine closer to flu season,” she says. “And therefore, we're able to better match it with the circulating viruses avoiding vaccine mismatch.”
Welch says that mRNA vaccines could also solve for another problem that can arise with traditional vaccines: something called “egg-adapted changes.” Because the flu virus can change over time, she says, when viruses are grown in eggs as the foundation of a flu vaccine, it can impact how well the vaccine will work once it's ready. The egg itself can introduce changes to the virus used in the vaccine that make it different from the virus that is in circulation.6 It may result in antibodies that aren’t as effective at fighting against the latest incarnation of the virus.7
Another possible benefit: There’s also the potential for the human body to develop strong responses with mRNA vaccines, says McLaughlin. The body develops “neutralizing antibodies” that attack foreign invaders. The body also receives a boost in “cellular immunity,” which refers to how the body responds to and kills cells if they become infected.
“The ability to activate both arms of the immune system could allow for preventing both symptomatic illness—tamping down spread of infection—and also preventing severe illness,” says McLaughlin.
The facts about flu
Flu can bring on a sore throat, runny nose, and cough. In these ways, it might feel similar to a cold. It can also bring on a fever, chills, aches, and pains and can lead to potentially life-threatening complications.8 Each year, 12,000 to 52,000 die from flu, and 140,000 to 710,000 spend time in the hospital.9 What's more, it’s a virus that disproportionately impacts racial and ethnic minorities. Compared with white Americans, Black Americans are 1.8 times more likely to be hospitalized for flu and Latino and Indigenous Americans are 1.2 and 1.3 times more likely.10
The best way to protect yourself from influenza is by getting vaccinated, according to the Centers for Disease Control and Prevention.11 And yet, that, too, presents challenges. Even when currently-available vaccine strains match circulating influenza virus strains well, they reduce the risk just 40% to 60%. Those numbers decrease when strains aren’t as well-matched.12 And those who face the greatest risk of developing severe illness or dying from flu are also the least likely to get vaccinated since racial and ethnic minority communities in the U.S. are vaccinated at lower rates,10 and vaccine clinical trials often lack diversity.13
Testing the mRNA flu vaccine candidate
In July, 2022, Pfizer reported positive clinical results in its Phase 2 trial of the mRNA flu vaccine candidate. “The data from that trial suggest a strong T-cell immune response in addition to good safety and tolerability in trial participants," says Welch. T-cells are white blood cells that recognize and defend against familiar germs.14
In September 2022, Pfizer began work to recruit 25,000 volunteers ages 18 and older to participate in the Phase 3 clinical trial for the mRNA flu vaccine. One goal of the trial is to include participants from a variety of age groups and backgrounds, in order to best reflect real-world diversity.
Welch says that in particular, she hopes to see a strong representation among minorities. “We want the results to be equitable. We want them to be generalizable. In order to know that the vaccine being developed works and is relevant to people like myself, as a minority, we have to participate in the trial,” she says. “To move the science forward, we need diverse participation and robust participation.”
The potential for mRNA vaccines
Though the discovery and early research into mRNA dates back to the 1960s, its application to vaccine development is still in its infancy. In fact, McLaughlin compares the potential for messenger RNA to the early days of the Internet. “We didn’t realize what all the applications of the Internet were going to be,” he says.
Because mRNA can tell our bodies what types of proteins to make, the sky may be the limit when it comes to its potential to protect or even improve health. We could see application of mRNA in cancer treatments, rare disease therapies, gene editing, or other areas, says Welch.
“I think we are just on the tip of what we'll be able to do in the future with this technology,” she says.
In many ways, the pandemic tested a hypothesis for mRNA vaccines: Could scientists quickly and safely develop and manufacture safe and effective vaccines? And could the regulatory authorities efficiently approve them? The answers were yes and yes. That, too, opens up a world of possibilities.
“What this pandemic showed through the lens of new mRNA technology is that science can help solve some of the world's largest problems. It can help improve lives. It can make sure that things stay normal or get back to normal,” says McLaughlin. “The phrase that we always use at Pfizer is ‘Science will win.’”
References
1 Johns Hopkins Bloomberg School of Public Health. The Long History of mRNA Vaccines. Available at https://publichealth.jhu.edu/2021/the-long-history-of-mrna-vaccines. Oct. 6, 2021. Accessed Sept. 8, 2022.
2 CDC. Selecting Viruses for the Seasonal Influenza Vaccine. Available at https://www.cdc.gov/flu/prevent/vaccine-selection.htm. Page last reviewed March 11, 2022. Accessed Sept. 7, 2022.
3 CDC. How Influenza (Flu) Vaccines Are Made. Available at https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm. Last reviewed Aug. 31, 2021. Accessed Sept. 6, 2022.
4 Interview with Verna Welch, September 1, 2022.
5 CDC. Understanding mRNA COVID-19 Vaccines. Updated July 15, 2022. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html. Accessed Sept. 6, 2022.
6 Vaccine. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Available at https://www.sciencedirect.com/science/article/pii/S0264410X20307945. Aug. 27, 2020. Accessed Oct. 25, 2022.
7 CDC. Cell-based Flu Vaccines. Available at https://www.cdc.gov/flu/prevent/cell-based.htm. Page last reviewed Sept. 2, 2022. Accessed Sept. 8, 2022.
8 CDC. Flu Symptoms and Complications. Available at https://www.cdc.gov/flu/symptoms/symptoms.htm. Last reviewed Aug. 25, 2022. Accessed Sept. 7, 2022.
9 CDC. Disease Burden of Flu. Available at https://www.cdc.gov/flu/about/burden/index.html Last reviewed Jan. 7, 2022. Accessed Sept. 7, 2022.
10 CDC. Flu Disparities Among Racial and Ethnic Minority Groups. Available at https://www.cdc.gov/flu/highrisk/disparities-racial-ethnic-minority-groups.html. Last reviewed Nov. 9, 2021. Accessed Sept. 7, 2022.
11 CDC. People at Higher Risk of Flu Complications. Available at https://www.cdc.gov/flu/highrisk/index.htm. Last reviewed Sept 6,2022. Accessed Sept. 6, 2022.
12 CDC. Vaccine Effectiveness: How Well do the Flu Vaccines Work? Available at https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm. Last reviewed Aug. 25, 2022. Accessed Sept. 7, 2022.
13 JAMA Network Open. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. 2021. Available at https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2776562. Feb. 18, 2021. Accessed Sept. 8, 2022.
14 CDC. Understanding How Vaccines Work. Available at https://www.cdc.gov/vaccines/hcp/conversations/understanding-vacc-work.html. Page last reviewed May 23, 2022. Accessed Sept. 8, 2022.
![]()